SOURCES FOR THE PODCAST
MOON LIVING
HOW COULD WE LIVE ON THE MOON?
Source: Institute of Physics iop.org
Settlers on the moon won’t be able to carry all their rations and resources on their rockets. It would be too heavy.
- Job #1: Making Breathable Air. NASA has already developed robots capable of doing this.
- Job #2: Find Water on the Moon. The Moon has no liquid water but in 2018 NASA confirmed it does exist on the surface in ice form. Rovers could find, drill, and recover this ice.
- Job #3: Settlers would need to bring seeds and earthworms from Earth. The worms would help to create a sustainable lunar agricultural ecosystem.
- Job #4: Create a long term power supply and permanent shelter. The lunar soil contains all the materials needed to build solar panels. The panels would need to be placed at a high point of one of the Moon’s poles. Settlers could come with inflatable or expandable structures also.
SOURCE FOR HYDROPONICS
Hydroponics
From Wikipedia, the free encyclopediaJump to navigationJump to search"Hydroponic" redirects here. For the 311 album, see Hydroponic (EP).Look up hydroponics in Wiktionary, the free dictionary.NASA researcher checking hydroponic onions (center), Bibb lettuces (left), and radishes (right)
Hydroponics[1] is a type of horticulture and a subset of hydroculture which involves growing plants, usually crops, without soil, by using water-based mineral nutrient solutions in aqueous solvents. Terrestrial or aquatic plants may grow with their roots exposed to the nutritious liquid or in addition, the roots may be physically supported by an inert medium such as perlite, gravel, or other substrates.[2] Despite inert media, roots can cause changes of the rhizosphere pH and root exudates can affect rhizosphere biology and physiological balance of the nutrient solution by secondary metabolites.[3][4][5]
The nutrients used in hydroponic systems can come from many different sources, including fish excrement, duck manure, purchased chemical fertilizers, or artificial nutrient solutions.[6]
Plants are commonly grown hydroponically in a greenhouse, on inert media, include tomatoes, peppers, cucumbers, strawberries, lettuces, and cannabis, usually for commercial use, and Arabidopsis thaliana, which serves as a model organism in plant science and genetics.[7]
Hydroponics offers many advantages, notably a decrease in water usage in agriculture. To grow 1 kilogram (2.2 lb) of tomatoes using intensive farming methods requires 400 liters (88 imp gal; 110 U.S. gal) of water;[citation needed] using hydroponics, 70 liters (15 imp gal; 18 U.S. gal); and only 20 liters (4.4 imp gal; 5.3 U.S. gal) using aeroponics.[8] Since hydroponics takes much less water to grow produce, it could be possible in the future for people in harsh environments with little accessible water to grow their own food.[9]
Contents1History2Techniques2.1Static solution culture2.2Continuous-flow solution culture2.3Aeroponics2.4Fogponics2.5Passive sub-irrigation2.6Ebb and flow (flood and drain) sub-irrigation2.7Run-to-waste2.8Deep water culture2.9Rotary3Substrates (growing support materials)3.1Rock wool3.2Expanded clay aggregate3.3Growstones3.4Coconut Coir3.5Rice husks3.6Perlite3.7Vermiculite3.8Pumice3.9Sand3.10Gravel3.11Wood fiber3.12Sheep wool3.13Brick shards3.14Polystyrene packing peanuts4Nutrient solutions4.1Inorganic hydroponic solutions4.2Organic hydroponic solutions4.3Additives4.4Tools4.5Mixing solutions5Additional improvements5.1Growrooms5.2CO2 enrichment6See also7References
A SOURCE FOR WATER
Gas Cracker
From Wikipedia, the free encyclopediaJump to navigationJump to search
A gas cracker is any device that splits the molecules in a gas or liquid, usually by electrolysis, into atoms. The end product is usually a gas. A hydrocracker is an example of a gas cracker. In nature, molecules are split often, such as in food digestion and microbial digestion activity. A gas cracker device splits the molecule at a rate much greater than that normally found in nature. In science and industry, gas crackers are used to separate two or more elements in a molecule. For example, liquid water, or H
2O, is separated into hydrogen and oxygen gases. This is not to be confused with the splitting of the nucleus (nuclear power).
Contents1Gas cracker2Other methods3References3.1See also3.2Sources3.3CitationsGas cracker[edit]
Petrochemicals are usually manufactured in large scale from petroleum feed stocks using fluid catalytic cracking. Naphtha, natural gas, refinery off-gas and gas from cokers and thermal crackers are good sources. Thus natural gas is one of the most wanted feed stocks for petrochemicals production. The thermal cracking of natural gas proceeds at very high temperature resulting in olefins (Mostly ethylene/propylene). The temperature in a gas cracker exceeds 1000°C. For ultimate decomposition of gas into elements more than 1500 °C is required. Thus, acetylene/carbon black production encounters such high temperatures. Usually oxy-combustion methods are used for attaining such high temperatures. BASF burners/Kellog burners are available in the market.
Other methods[edit]
Further electro cracking or plasma cracking methods are also available.
References[edit]See also[edit]Plasma polymerizationPyrolysisGas-phase ion chemistrySources[edit]Fahad H. Falqi, 2009, The Miracle of Petrochemicals: Olefins Industry : an In-depth Look at Steam Crackers.Herman F. Mark, 2013, Encyclopedia of Polymer Science and Technology, Concise.William L. Leffler, 2014, Natural Gas Liquids: A Nontechnical Guide.Citations[edit]Categories: ElectrolysisGas technologies
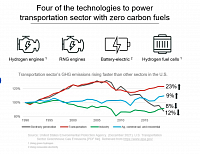
Hydrogen and hydrogen engines have been receiving a lot of attention in business circles, in the media, and in government. With good reason—the need to reduce global greenhouse gas emissions and reach destination zero is greater than ever. And hydrogen fuel is one of the most promising carriers of non-fossil energy. In the electricity sector, power-to-hydrogen and hydrogen-to power-technologies such as hydrogen combustion turbines are rapidly developing. In the transportation sector, initial attention was focused on fuel cell hydrogen electric vehicles, or FCEVs. More recently, hydrogen vehicles powered by internal combustion engines are also receiving increased attention, especially among medium and heavy-duty trucking applications. Hydrogen engines can enable your journey to destination zero using carbon-free hydrogen fuel as FCEVs, and use technology familiar to vehicle manufacturers, fleets and drivers.
ENVIRONMENTAL BENEFITS OF HYDROGEN ENGINES
Vehicles with hydrogen internal combustion engines can operate without any CO2 emissions coming from the hydrogen fuel, direct or indirect, depending on the source of the hydrogen used. Hydrogen produced by electrolysis using electricity coming from solar panels or wind turbines, for example, enables CO2-free driving. Additionally, hydrogen fuels do not release any particulate matter, carbon monoxide, or volatile organic compounds. Hydrogen engines do, however, have the potential to release some NOx, an atmospheric pollutant that can contribute to the haze sometimes observed above large cities during summer months. Aftertreatment systems are used to eliminate most NOx emissions. In the United States, converting medium and heavy-duty trucks to clean hydrogen would eliminate about a quarter of all greenhouse gas emissions from the transportation sector.
HYDROGEN ENGINES’ ROLE IN DESTINATION ZERO EMISSIONS
Hydrogen, obtained through renewable sources, is one of the zero-emissions fuels to power vehicles. Hydrogen engines offer a unique benefit to vehicle manufacturers and fleets among different low to zero carbon technologies. Hydrogen engines are built upon today’s modern and reliable internal combustion engines. For vehicle manufacturers, it is a familiar technology to use in their vehicle design and production. Similarly, for fleets, it is a familiar technology to operate, maintain, troubleshoot, and service.
TRANSITIONING INTO HYDROGEN INTERNAL COMBUSTION ENGINES
Hydrogen engines are reliable, have familiar technology, and deliver environmental benefits. These make the transition into hydrogen engines operationally and economically viable. Meanwhile, two areas often come to mind as potential challenges in transitioning into hydrogen engines. The first is on-board storage. Hydrogen vehicles require economical ways to store hydrogen onboard. Cummins Inc. has recently formed a joint venture with NPROXX, a leader in hydrogen storage and transportation for hydrogen storage tanks. This joint venture will provide hydrogen and compressed natural gas storage products for on-highway and rail applications. The second is fueling infrastructure. Hydrogen cars and trucks can only be driven to the extent hydrogen is available. This is where trucking becomes a great initial use-case for hydrogen engines – more about this in the next section.
APPLICATIONS TO SEE HYDROGEN ENGINES FIRST
What type of vehicle should we expect to see adopt hydrogen engines in large numbers? Contrary to what has been believed for decades, it is not likely to be personal cars—battery electric technology seems to be the better choice for that application. Medium and heavy-duty applications are much more likely to turn to hydrogen fuel cells or hydrogen engines. It is likely in the coming decade, buses and long-haul trucks running on hydrogen are going to become a common sight. These come complementary to battery-electric buses and trucks that also are economically and operationally viable on certain mission profiles and applications. Off-road, construction equipment, agricultural machinery, and even ships featuring a hydrogen engine are also likely to become common. These are most likely going to be the applications that are hard to electrify due to their use-cases and mission profiles. Power generation applications are another use-case for the near-term application of hydrogen engines to produce electricity.
HYDROGEN ECONOMY TO BE KICKSTARTED BY HYDROGEN ENGINES
The hydrogen economy is a society-wide solution to global warming and to the exhaustion of fossil fuel. In the hydrogen economy, fossil fuels are replaced by hydrogen produced from renewable sources. One of the main challenges preventing progress in this direction is of circular nature. Mainstream hydrogen use can start once hydrogen fuel is widely available; and, hydrogen fuel will be widely available once there is mainstream use for it. Good news; there are applications where use of hydrogen engines is viable in the absence of an extensive network of hydrogen fueling stations. Long-haul trucking powered by hydrogen engines, for example, is possible with only a few hydrogen stations placed along the main shipping routes. It is possible to see hydrogen engine applications initiating a virtuous cycle leading to a greater availability of hydrogen and thus to more hydrogen applications.
HYDROGEN ENGINES AND FUEL CELLS
Hydrogen engines and hydrogen fuel cells are very different technologies that achieve a similar function—powering a vehicle using hydrogen. They are two complementary technologies that serve different applications and respond to different end user requirements. Fuel cells are a new and advanced technology. Hydrogen engines are just modified internal combustion engines. The hydrogen fueling infrastructure developed for the applications of one can serve the applications of the other. And, any development towards more economical onboard hydrogen storage is entirely applicable to both.
Source:. https://www.cummins.com/engines/hydrogen-engines?fbclid=IwAR1q_Ca6HW0hAiG9mR8V_U_7SXB6cdQp3tEMXroxnYvyJlHcB7CEufvO6Dc
Hydrogen is the simplest and most abundant element in the universe. It is a major component of water, oil, natural gas, and all living matter. Despite its simplicity and abundance, hydrogen rarely occurs naturally as a gas on Earth. It is almost always combined with other elements. It can be generated from oil, natural gas, and biomass or by splitting water using renewable solar or electrical energy.
Once hydrogen is produced as molecular hydrogen, the energy present within the molecule can be released, by reacting with oxygen to produce water. This can be achieved by either traditional internal combustion engines, or by devices called fuel cells. In a fuel cell, hydrogen energy is converted directly into electricity with high efficiency and low power losses.
Hydrogen, therefore, is an energy carrier, which is used to move, store, and deliver energy produced from other sources.
Learn more about:
Or read more about EERE's hydrogen technologies research.